Gelatin Transformation (Istihala)
“In Science and Fiqh”
Muslims community represents about 30% or about 2 billion of the world population. The majority of Muslims are from Asia Pacific which represent 70% and Middle-East, 20%. These numbers are expecting to increase 2.7 billion in 2030. This means that the demand for halal products will be increased.
Food industry is one of the main concerns in Muslims community. The rapid development in food technology results the emergence of various food products and food ingredients in market. Many food ingredients are produced from doubtful sources. In addition, lack of awareness from Muslim consumers might lead to the difficulties in choosing purely halal food products in market. One of the most controversial issues in food industry in Muslims world is gelatin based food products.
Gelatin is a mixture of peptides and proteins produced by partial hydrolysis of collagen extracted from the skin, bones, and connective tissues of animals such as domesticated cattle, chicken, pigs, and fish.
Gelatin, one of the most popular biopolymers, is widely used in food, pharmaceutical, cosmetic, and photographic applications because of its unique functional and technological properties. In food industry gelatin is utilized in confections (mainly for providing chewiness, texture, and foam stabilization), low-fat spreads (to provide creaminess, fat reduction, and mouthfeel), dairy (to provide stabilization and texturization), baked goods (to provide emulsification, gelling, and stabilization), and meat products (to provide water-binding) (Johnston-Banks, 1990; Schrieber & Gareis, 2007).
The global demand for gelatin has been increasing over years. Recent report indicate the annual world out-put of gelatin is nearly 326,000 tons, with pig skin derived gelatin accounting for the highest (46%), followed by bovine hides (29.4%), bones (23.1%) and other sources (1.5%) (Karim and Bhatt, 2009). In Europe, about 80% of edible gelatin is produced from pig skin (Boran and Regenstein, 2010).
Islam forbade it followers to consume haram and doubtful foods. It is stated in Al-Quran:
(Forbidden to you (for food) are: dead meat, blood, the flesh of swine, and that on which hath been invoked the name of other than Allah. that which hath been killed by strangling, or by a violent blow, or by a headlong fall, or by being gored to death; that which hath been (partly) eaten by a wild animal; unless ye are able to slaughter it (in due form); that which is sacrificed on stone (altars); (forbidden) also is the division (of meat) by raffling with arrows: that is impiety). (Al-Maidah, 5:3)
Jurists agree in consensus that pork and all its parts are Haram. The scholar Alfakhr Alrazi said (Islamic nation agreed in consensus that all the pork parts is haram, and Allah mentioned only for meat only because generally most utilization related to meat ). Al-Kurtubi also said (Islamic nation agreed in consensus that all the pork lard is haram). Ibn Hazm said :” it is forbidden to eat anything belong to pig neither its meat, nor lard, nor skin, nor connective tissues, nor brain, nor bone, nor milk, nor hair……etc.
Generally, Muslim jurist agreed that gelatin derived from slaughtered and permitted animals is halal. However, there is argument on gelatin that is derived from pig and carrion. The halal and haram sources of this matter have been debated among Muslim jurists. Some of them agreed that gelatin extracted from the prohibited sources is haram. Whereas the other opinion supported the idea that gelatin from haram sources is halal because it does already undergo Transformation (Istihalah) process (Hammad, 2004).
Istihalah, from the Fiqh point of view, is defined as “changing the nature of the defiled or forbidden substance to produce a different substance in name, properties and characteristics. Istihalah also can be defined as a complete transformation occurred physically and chemically (Aizat & Radzi, 2009).
The scholar (Inb Abdeen) said that: “transformation is changing and conversion of the material nature”. Then he gives examples about transformation, and then he said:” in all of those examples there is change of the material nature to new nature, and it is not only conversion of characteristics.
Dr. Hamed jami mentioned in the 9th medical fiqh Symposium about the criteria of transformation, and he said: the transformation criteria from Islam point of view mean the complete conversion of the material nature, and so all its nature, characteristics and properties must convert, and so the transformed material must be not similar to the original material, and it must has new independent nature, another characteristics, and new name.
If we take the most famous example about transformation, which is transformation of wine (ethanol) to vinegar (acetic acid), we can notice that the original material (ethanol) belongs to alcohols group, but the transformed material (acetic acid) belongs to acids group. This mean there is complete conversion in physical and chemical properties, and there is change of the material nature to new nature, and it is not only conversion of characteristics.
Can we consider the conversion of collagen into gelatin is transformation?!!
Actually, what happened to collagen is changing in some its chemical and physical properties and it is not transformation, and the principle of transformation did not achieved here, and we will explain that in details.
Gelatin production:
There are two basic types of gelatin:
Gelatin type A: The raw material (mostly pig skin) is subjected to a 24-h conditioning process. This involves treatment with acid. After this, the gelatin can be extracted.
Gelatin type B: The raw material (either ossein or bovine hide) is subjected to several weeks of treatment with alkali. The collagen then become soft can be extracted using warm water.
The manufacturing processes of gelatin consist of three main stages:
1-Pretreatments: to make the raw materials ready for the main extraction step and to remove impurities which may have negative effects on physio chemical properties of the final gelatin product,
2-Extraction: The main extraction step which is usually done with hot water or dilute acid solutions as a multi-stage extraction to hydrolyze collagen into gelatin, and finally,
3- Refining and recovery: The refining and recovering treatments including filtration, clarification, evaporation, sterilization, drying, rutting, grinding, and sifting to remove the water from the gelatin solution, to blend the gelatin extracted, and to obtain dried, blended and ground final product.
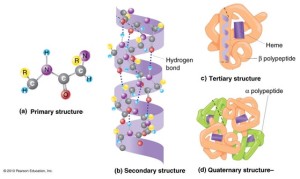
The treatment of collagen with acids and alkaline causes changes in the tertiary structure of the protein by dissociation of some hydrogen bonds between proteins chains, and breaking some poly peptides bonds. This gives proteins with low molecular weight and water soluble. We have to know that there are no changes in the primary and secondary protein structure. In other word, there are no changes in the helix protein structure and there no changes in the amino acids sequence which responsible for chemical composition. In addition to, we can recognize the kind of the animal by analysis of gelatin.
For more understanding of protein we should know the protein structure:
Primary structure: comes from the sequence of amino acids in polypeptides. This sequence responsible for distinguish protein from another, and responsible for determining general and physical properties of the protein.
Secondary structure is defined by the patterns of hydrogen bonds between backbone amino and carboxyl groups. Secondary structure gives the helix structure to the protein.
Tertiary structure is the final specific geometric shape that a protein assumes. This final shape is determined by a variety of bonding interactions between the “side chains” on the amino acids proteins fold into one or more specific spatial conformations.
Conversion of collagen into soluble gelatin is due to the cleavage of a number of intra- and intermolecular cross-linking bonds in collagen. As a result, the gelatin obtained generally has molecular weights lower than native collagen (Asghar & Henrickson, 1982). Insoluble native collagen must be pre-treated before it can be converted into a form suitable for extraction, which is normally done by heating in water at temperatures higher than 45°C. A chemical pre-treatment will break non-covalent bonds so as to disorganize the protein structure, thus producing adequate swelling and collagen solubilization (Stainsby, 1987). Subsequent heat treatment cleaves the hydrogen and covalent bonds to destabilize the triple-helix, resulting in helix-to-coil transition and conversion into soluble gelatin. (Djabourov, Lechaire, & Gaill, 1993; Gomez-Guillen et al., 2002).
The difference between collagen and gelatin
Collagen molecules are composed of three α-chains intertwined in the so-called collagen triple-helix. This particular structure, which mainly stabilized by intra- and inter-chain hydrogen bonding, is the product of an almost continuous repeating of the Gly-X-Y- sequence, where X is mostly proline and Y is mostly hydroxiproline. (Asghar & Henrickson, 1982).
The amino acid composition and sequence in gelatin are different from one source to another but always consists of large amounts of glycine, proline and hydroxyproline (Gilsenan, and Ross-Murphy, 2000). It is repeated with typical sequence, Gly-X-Y where glycine is the most abundant amino acid in gelatin; X and Y are mostly proline and hydroxyproline, respectively. 25% of dry gelatin contains proline and hydroxyproline that stabilize its structure (Russell et al., 2007). The molecular compositions of collagen and gelatin are almost identical (Djabourov M, Lechaire JP, Gaill F 1993). According to Schrieber & Gareis, (2007), the composition of collagen encompasses all 20 amino acids. Glycine, proline and hydroxyproline are the largest numbers of amino acid exist in gelatin.
The chemical properties of gelatin are affected by amino acid composition, which is similar to that of the parent collagen, thus influence by animal’s species and type of tissues (Zhou and Regenstein, 2006).
Conclusion
As a result, the argument which claims that Istihalah process occurred on gelatin after undergo conversion process cannot be accepted, and according to the Juristic rule: “Certainty may not be disproved by doubt”. Since the pork, carrion and their parts are Najis (unclean), therefore gelatin extracted from pig and carrion is unclean and not allowed to use it in food and cosmetics.
REFERENCES
Asghar, A., & Henrickson, R. L. (1982). Chemical, biochemical, functional, and nutritional characteristics of collagen in food systems. In: Advances in food research, vol.28. London: Academic Press. pp. 232–372.
Boran, G., & Regenstein, J. M. (2010). Chapter 5. Fish gelatin. Advances in Food and Nutrition Research, 60, 119–143.
Collagen as food. Advances in meat research, vol. 4 (pp. 209–222). New York: Van Nostrand Reinhold Company, Inc.
Djabourov, M., Lechaire, J., & Gaill, F. (1993). Structure and rheology of gelatin and collagen gels. Biorheology, 30, 191–205.
Gilsenan, P. M., & Ross-Murphy, S. B. (2000). Rheological characterizations of gelatins from mammalian and marine sources. Food Hydrocolloids, 14,191-195.
Gomez-Guillén, M. C., Turnay, J., Fernndez-Diaz, M. D., Ulmo, N., Lizarbe, M. A., & Montero, P. (2002). Structural and physical properties of gelatin extracted from different marine species: a comparative study. Food Hydrocolloids, 16(1), 25-34.
Johnston-Banks, F. A. (1990). Gelatin. In P. Harris (Ed.), Food gels (pp. 233–289). NewYork: Elsevier Applied Sciences.
Kami Büyüközer H. and Sakr A. (2011). Jelatin, Jelatin Uretimi
Karim, A.A. and R. Bhat, 2009. Fish gelatin: Properties, challenges and prospects as an alternative to mammalian gelatins. Food Hydrocolloid, 23: 563-576
J. Aizat & C.W. J. W. M. Radzi. Theory of Istihalah In Islamic and Science Perspective: Application For Several Food Processing Products. Jurnal Syariah. 2009, 17, (1): 169-193
Hammad. al-Mawad al-Muharramah wa al-Najisah fi al-Ghiza’ wa al-Dawa’ bayna al-Nazariyyah wa al-Tatbiq. Syria. Dar al-Qalam. 2004. pp. 16.
Schreiber, H. Gareis. The raw material ‘Ossein’. In. R. Schreiber and H. Gareis.(eds.). Gelatine Handbook-Theory and Industrial Practice. Weinham, Wiley-VCH. 2007. pp. 63-71
Russell, J. D.; Dolphin, J. M. and Koppang, M. D. 2007. Selective analysis of secondary amino acids in gelatin using pulsed electrochemical detection. Analytical Chemistry 79: 6615-6621.
Stainsby, G. (1987). Gelatin gels. In A. M. Pearson, T. R. Dutson, & A. J. Bailey (Eds.),
Zhou, P. and Regenstein, J. M. 2006. Determination of total protein content in gelatin solutions with the Lowry or Biuret Assay. Journal of Food Science 71 (8), 474-479.